Introduction
Recent Research Work: A Brief Review
Background of the Research Work
Methodology of Geopolymer Paste and Mortar Using Alternative Cementitious Materials
Materials Used
Preparation of Alkaline Solution
Mix Proportion
Process of batching, mixing, and casting molds
Standard Consistency
Final Setting Time
Compressive Strength
Scanning Electron Microscopy (SEM) and X-Ray Diffraction (XRD) Analysis
Result with Discussion
Conclusion
Introduction
The global output of OPC has been steadily increasing, with annual increases ranging from 8 to 10%. Unfortunately, this increase has come at a high cost to the environment, contributing around 8% of global Carbon Dioxide (CO2) emissions [1]. As a outcome , the construction industry is aggressively seeking ways to become less reliant on OPC in order to create more environmentally friendly infrastructure [2]. The use of byproducts from many industries, including as GGBS, FA and RSH,has the potential to reduce the amount of cement used [3]. Researchers have conducted extensive study on a variety of cementitious materials, including varied amounts of cement substitution, in an attempt to develop environmentally acceptable alternatives like green or geopolymer concrete [4]. This is mostly owing to the numerous pozzolanic reaction mechanisms at work, which are distinct from how they operate in normal concrete [5]. Researchers were looking for new choices, therefore they looked into cement-free binders, which led to the creation of “geopolymer.” This could provide a more environmentally friendly option for construction materials, fire protection, reinforced composites, and even satisfying the needs of the nuclear and chemical industries [6]. FA, a coal-fired power plant by product, accounts for between 75 and 80 percent of global ash output [7].
Davidovits was the first to introduce the term “geopolymer” to explain how silicon (Si) and aluminum (Al) react chemically in alkaline solutions with FA to produce inorganic binders. Geopolymer is formed as a result of this process [8]. Source materials containing a high concentration of silicon and aluminum, as well as alkaline liquids, are critical components of geopolymers. These elements are also present in garbage [9]. As a result, the geopolymer technology not only reduces CO2 emissions but also converts industrial waste residuals containing aluminum silicate into environmentally friendly building materials [10]. Fly ash (FA) has been used as the primary alumina-silicate source material in various studies on geopolymer concrete [11]. To increase the mechanical qualities of geopolymer concrete, the material must be cured using the suitable processes [12]. Furthermore, submitting geopolymer concrete to elevated temperatures could result in the production of larger pores, an raise in the number of pores, and negative effects on the material’s qualities [13]. Compressive strength can be increased by raising the curing temperature within a certain range; however, temperatures that are excessively high may have negative consequences. The use of GGBS has been found to accelerate the rate of solidification of geopolymer paste, reducing the amount of time required for deployment and compaction [14].
RHA is an amorphous supplementary material produced by rice mills from agro-industrial waste [15]. When appropriate additives are applied, using RHA instead of fly ash, GGBS, or red mud has been proven to improve the mechanical and microstructural qualities of conventional GPC [16]. Furthermore, the use of GGBS, a by product of iron and steel production in blast furnaces, has been found to significantly enhance the power of geopolymer concrete (GC). The polymerization process has a better influence on the power and microstructure of GC [17]. Despite extensive study on fly ash-based GPC, it is still necessary to cure it at a high temperature to accelerate the process of polymerization and make the concrete stronger [18]. However, because of RHA’s high alumina and silica concentration, it can be utilized to create novel concrete combinations and geopolymers [19]. Despite these advances, there has been very little research on the combined effect of GGBS and RHA on GPC. However, it is impossible to reject the prospect of using these materials to develop sustainable construction processes and reduce the total environmental effect of the construction industry [20].
The contributions can be shortened as follows:
•The materials used to examine the correlation between the concentration of AAS and the qualities of mortar, considering different proportions of the constituent materials.
•The approach takes into consideration differences in the proportions of GGBS, RHA, and FA and various tests, such as final setting time, standard consistency and compressive strength, are considered.
•It looks at the fluctuation in the Na2SiO3 to NaOH ratio, specifically at 1.5 and 2.0 and the molarity of NaOH in AAS was changed at 8, 12, and 16 M concentrations.
•Compressive strength testing is performed in two different curing environments: outdoor curing and oven curing.
The rest of this paper is arranged as: Part 2 analyses recent research work and the background of recent research work. Part 3 demonstrates the methodology of work. Part 4 describes the results and discussion. The manuscript is concluded in Part 5.
Recent Research Work: A Brief Review
Many research works have previously existed in the literature which was based on physical and mechanical characteristics of geopolymer paste and mortar using alternative cementitious materials with various techniques and aspects. Few of the works were reviewed here.
Liang et al. [21] have suggested the impact of substituting up to 40% of the metakaolin (MK) with on the microstructures and stability over heat of MK-based geopolymers. The outcomes demonstrate that the RHA fractions of aluminosilicate participated in the geopolymerization’s acid-base reaction, which aided in the formation of more gels. The primary cause of the optimization of thermal stability of geopolymers was the filling effects of RHA and enhancement of gel phases, which polished the pores of the material. Samantasinghar and Singh [22] have developed that industrial activities generate massive volumes of byproducts, which have a adverse effects on the environment. The current cement manufacturing process was energy intensive and creates massive volumes of greenhouse gases. Geopolymers were a new category of green materials that have the potential to replace traditional cementitious materials. The features of binary blends of slag and FA -based geopolymer paste and mortars activated with sodium hydroxide, both in their fresh and hardened states, were described. Additionally, during the cycle of setting and hardening, the chemical byproducts, bonding, and changes in the microstructural were studied. Faried et al.[23] have presented that the use of GPC was regarded as essential for mitigating environmental hazards by reducing cement use. Therefore, a step-by-step mix design method for GPC based on GGBS was created with the goal of achieving convenient workability and compressive strength (CS) at a reasonable cost by utilizing various variables. Furthermore, giving design charts under varied curing conditions. The mix design process was depicted in the form of a flow diagram, which was clarified with the help of an illustration.
Gupta [24] had indicated that the aim of the study was to replace 60% of the slag with silica fume in order to produce a GGBS -based geopolymer composite. A catalytic liquid system (CLS) activated the aluminosilicates that make up slag and silica fume. NaOH and Na2SiO3 solutions were combined to form the catalytic liquid system. The strength in compression of the created geopolymer composite was evaluated at the ages of 7, 28, and 56 days. Three systems of catalytic liquid were created by varying the Na2SiO3 and NaOH ratios. Catalytic liquid system with geopolymer binder ratios of 0.3, 0.35, and 0.4 were employed in the current investigation. Nine different combinations of the a liquid catalyst system and the CLS/GB ratio were employed to produce 81 samples of the geopolymer (GP)composite. Kaya and Koksal [25] have presented that the Geopolymer mortars were created by replacing high calcium FA with cement at 5%, 10%, 15%, 20%, 25%, and 100%. The activator was sodium hydroxide, and the sodium/binder weight ratios in mortar mixtures were 10%, 12%, 14%, 16%, 18%, and 20%. Specimens maintained at room temperature underwent tests for unit weight, water absorption, apparent porosity, and ultrasonic pulse velocity. Tests on specimens maintained at room temperature and subjected to temperatures for residual mortar performance were conducted for flexural and compressive strength. Bellum et al. [26] have suggested that because of its environmentally friendly character, the alkali-activated geopolymer binder was a promising alternative to OPC binders. Because of the massive amounts of CO2 that cement manufacture releases into the atmosphere and the lack of natural resources, GPC has to be introduced into the building sector. In this investigation, the mechanical characteristics of Geopolymer GPC cured in ambient circumstances using fly ash and GGBS were investigated. In order to predict the values, codes and previously published literature were compared with experimental data on Modulus of Elasticity (MOE) values. Lianasari and Syafig et al. [27] have developed that the primary component of concrete was cement. For every ton of clinker, 0.869 tons of CO2 gas were created during the calcination process during cement manufacture. This process has an impact on global climate change. This circumstance gives rise to a new technology known as geopolymer concrete. Geopolymer concrete was made by replacing cement with a substance that reacts with an alkali activator. GGBS was the name given to blast furnace slag that comprises SiO2 and Al2O3. GGBS can react with an alkali activator to form a cement substitute. This study used GGBS to investigate the impact of the ratio of alkali activator on the mechanical characteristics of GPC [28, 29, 30].
Background of the Research Work
The review of the recent research work displays that Geopolymer paste and mortar were alternative cementitious materials that have gained popularity in the past few years due to their potential environmental benefits and superior mechanical qualities over typical Portland cement-based materials. Typically, geopolymer materials were created by mixing aluminosilicate precursors, such as FA or slag, with an solution for an alkaline activator. Depending on the composition and curing circumstances, the physical properties of geopolymer paste and mortar might vary. Geopolymer paste has a higher viscosity than standard cement paste, which might impair workability and ease of application. Geopolymer paste often has a longer setting time than Portland cement paste. Geopolymer materials can also increase other mechanical characteristics such as tensile strength, flexural strength and durability.Geopolymer paste and mortar mechanical characteristics might vary based on the formulation and curing circumstances. There weren’t many literary works introduced to address this problem, and the ones that were ineffective. These drawbacks and problems have motivated the study being done.
Methodology of Geopolymer Paste and Mortar Using Alternative Cementitious Materials
Materials Used
The geopolymer (GP) paste materials used in the manufacturing process have a substantial effect on the efficiency of the properties produced. In the production of GP paste, materials such as low-calcium FA (class F), GGBS, and RHA are used. Natural river sand is used in the manufacture of fine aggregates. As an AAS, a 1.5:2 mixture of Na2SiO3 solution and NaOH solution is used. AAS employs a range of sodium hydroxide molarities, including 8M, 12M, and 16M.
Fly Ash
The low calcium-based FA (Class F) acquired from the Thermal Power Plant was used as one of the binder materials. The physical property of FA such as specific gravity was found to be 2.25 and whereas fineness of fly ash was 360 (m2/kg).
Ground Granulated Blast Furnace Slag
Molten iron slag from a blast furnace was quenched in steam or water to produce the GGBS. It was discovered that the physical characteristics of GGBS were 400 m2/kg of fineness and 2.83 specific gravity.
Rice Husk Ash (RHA)
RHA is a type of agricultural waste that, depending on the activation of the mix, contains amorphous silica. RHA was found to boost tensile and flexural modulus while decreasing tensile strength elongation. The specific gravity of RHA was found to be 2.14, and the fineness of RHA was 192m2/kg. Table 1 lists the chemical compositions of FA, GGBS, and RHA.
Table 1.
Chemical compositions of FA, GGBS, RHA (% mass)
Fine Aggregate
The fine aggregate utilized was the organic river sand. The fine aggregate’s specific gravity is 2.64, its fineness modulus is 2.54, and its absorption of water is 1%.
Alkaline Liquid
The AAS is a mixture of Na2SiO3 and NaOH, mixed in the ratios of 1.5 and 2. One day before casting, the alkaline activator solution is made and kept in a dry location with little exposure to moisture.
Sodium silicate
Na2SiO3 is commonly called as liquid glass. It is made from SiO2 and Sodium oxide (Na2O). The mass ratio of SiO2 and Na2O is 2.61. The proportion of the constituents of the solution is as: Water makes up 58.5%, SiO2 is 30%, and Na2O is 11.5%.
Sodium hydroxide
White and crystalline, sodium hydroxide is caustic and absorbs moisture quickly until it dissolves. The most often used industrial alkali is sodium hydroxide.. It is also known as caustic soda. For instance, each liter of an 8M, 10M, or 12M NaOH solution contains 320 grams of solid NaOH (in the form of pellets), 400 grams of solid NaOH, or 480 grams of solid NaOH. The molecular weight of NaOH in this case is 40.
Preparation of Alkaline Solution
These experiments use a mixture of Na2SiO3 and NaOH. The goal is to see how the solutions change when different concentrations of NaOH (8M, 12M, and 16M) are added. It also wishes to learn what happens to the entire process when the amounts of NaOH and Na2SiO3 are raise from 1.5 to 2. These solutions are meticulously mixed and prepared the day before they are cast, and they are kept perfectly dry at all times. This precaution is necessary because the polymerization reaction generates a lot of heat while the solution is being created. The weight-to-weight (w/w) ratios are shown, and the amount needed is determined by the strength of the NaOH solution. The connection between NaOH and Na2SiO3 is the main emphasis. Sodium hydroxide, sometimes known as caustic soda or lye, is a highly powerful alkaline chemical. Complex silicate structures are created when these two components are mixed together due to a chemical reaction. The selection of many molarity levels, notably 8M, 12M, and 16M, is a critical variable.
Mix Proportion
The development process used was influenced by earlier research on FA-based geopolymer mortar (GPM), which serves as the established standard for GPM mix proportions.
Process of batching, mixing, and casting molds
The fine aggregate and binders, which include GGBS, FA, and RHA, are thoroughly mixed according to the required mix proportions, yielding a dry mix. To obtain a homogenous paste, the material is thoroughly stirred for 3-5 minutes. The moulds for compression testing of GPM cubes of dimensions 70.6 X 70.6 X 70.6 mm are lubricated on the inside surface to ensure simple removal as it prevents stick ability of GPM, which is highly bendable to steel.
Standard Consistency
Table 2 displays the typical consistency values for several binder combinations, namely FA, GGBS and RHA while keeping a consistent fly ash percentage of 50%. RHA is added to GGBS at various concentrations of 0%, 2%, 4%, 6%, 8%, and 10%. In addition, compare the various molarities, notably 8M, 12M, and 16M, to two separate alkaline solution ratios, 1.5 and 2.0. According to the statistics, the standard consistency values range from a minimum of 33% to a maximum of 42%.The aforementioned observation could have implications for the practicality and manipulating qualities of AAS-based materials. The impact of alkaline solution ratios and molarity on the consistency characteristics of AAS-based mixtures appears to be minor. The amount of GGBS and the presence of RHS, on the other hand, are important factors in determining these qualities. The findings have practical implications for developing AAS formulations to meet specific technical requirements, particularly in cases where consistency and workability are critical factors.
Table 2.
Standard consistency ofgeopolymer paste for varying NaOH molarities
Final Setting Time
The findings of the study contributed significantly to a better understanding of the intricate link between the molarity of NaOH in an alkaline solution and the eventual setting time of a product. The outcomes show that there is a considerable and continuous relationship between NaOH concentration and eventual setting time, notably at concentrations of 8M, 12M, and 16M. This shows that increasing the concentration of NaOH in an alkaline solution results in a longer time required for the substance to solidify. Table 3 displays the final GPC setting results. When the molarity ratio is adjusted from 1.5 to 2.0, an intriguing occurrence occurs: the ultimate setting time decreases unexpectedly. This behavior suggests the existence of a NaOH molarity range that is ideal for reaching the desired setting time. A rise in the fraction of RHA in the combination, on the other hand, has an independent effect. The setting time is significantly delayed when different quantities of GGBS are substituted with RHA, specifically 0%, 2%, 4%, 6%, 8%, and 10%.The results of this investigation show that including RHA in the mixture can increase the rate of setting. It should be noted, however, that an increase in the RHA percentage demands a bigger quantity of activated solutions. The overall elongation of setting times with higher NaOH molarity demonstrates the influence of the specific molarity ratio on setting times. The study emphasizes the necessity for a comprehensive comprehension of these interactions in order to progress the advancement of constructing materials that possess customized features.
Table 3.
Geopolymer paste’s final setting for various NaOH molarities
Compressive Strength
The composition of the binders in geopolymer mortars has a considerable crash on their CS. The binder contains fly ash, GGBS, and RHA in the scope of this inquiry. During the experimental period, a steady fly ash percentage of 50% was maintained while the amount of RHA was varied from 0% to 10%. RHA was replaced with GGBS. The obvious association between greater percentages of RHA and increased compressive strength is a key finding of experimental investigations. The observed phenomena are known as RHA’s distinct characteristics. The inclusion of tiny particles in RHA acts as supplemental reactive components, contributing to the geopolymer’s overall strength. Another key variable was the molarity (concentration) of the alkali activator solution, which typically contained NaOH and Na2SiO3. The three different molarities are 8M, 12M, and 16M. Experiments show that raising the molarity of NaOH increases the CS of geopolymer concrete by the same amount. Higher molarities lead to greater levels of alkali molecules, which explains this effect. They are essential in the process of dissolving alumina silicate minerals found in fly ash and GGBS, allowing for greater geopolymerization and increased strength. The investigation also included the examination of various Na2SiO3 to NaOH ratios, notably 1.5 and 2.0. The experimental results show that the Na2SiO3to NaOH ratio correlates with the CS of GPC . The role of sodium silicate in the formation of the geopolymer gel structure may be traced. An higher proportion of Na2SiO3to NaOH promotes geopolymerization, resulting in improved strength. Higher concentrations of RHA, higher molarities of NaOH, higher sodium silicate ratios, and higher curing temperatures all have a favorable impact on the final CS. The findings are noteworthy for the development of GPC mix designs because they have the potential to significantly improve mechanical qualities for a wide range of construction applications.
Scanning Electron Microscopy (SEM) and X-Ray Diffraction (XRD) Analysis
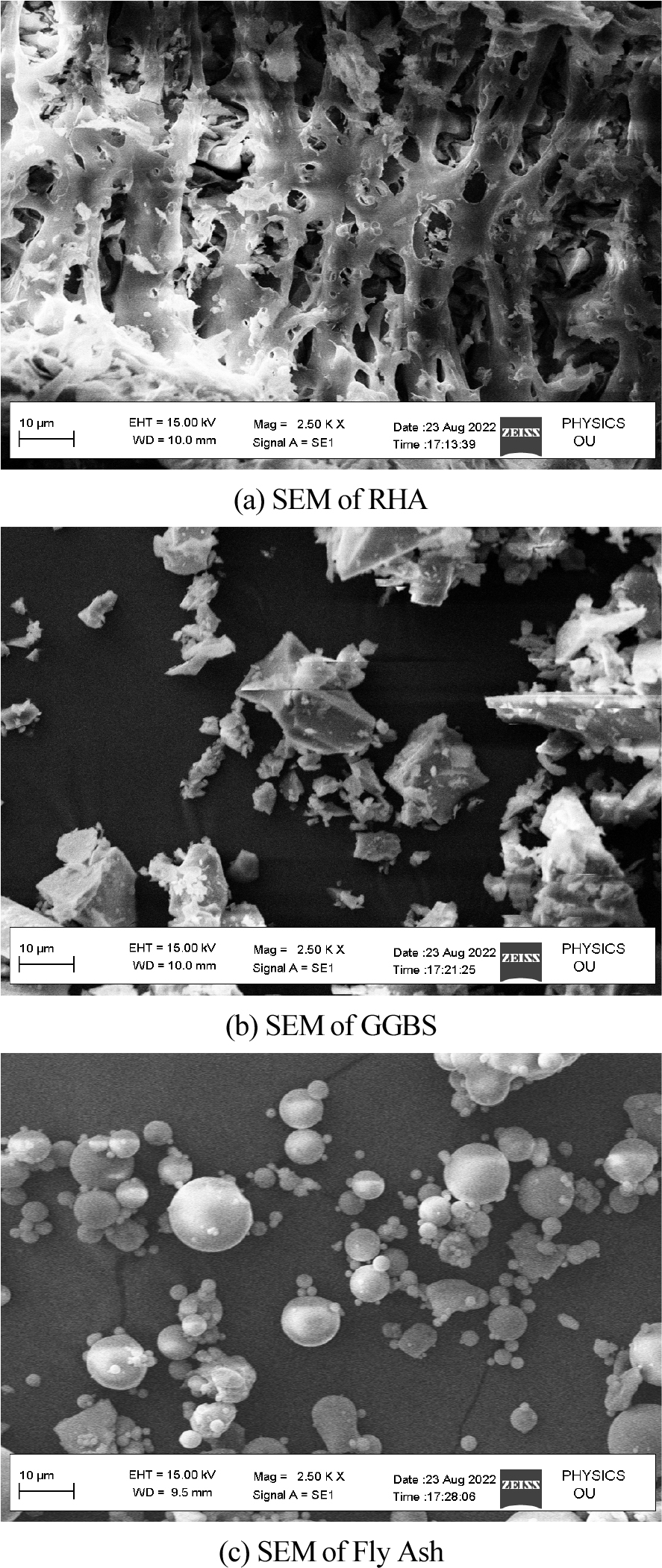
SEM was used to produce images of the RHA. RHA particles with amorphous morphologies, such as cristobalite and crystalline quartz, were found to be solid in nature. High-temperature processing yields crystalline RHA with amorphous forms. The uneven particle size was caused by the RHA’s high combustion temperature. The photographs of the SEM image (particle forms) of GGBS show that it has numerous shapes of various sizes for mixing procedures and geopolymer manufacture. The SEM study of class-F FA in the manufacture of a geopolymer demonstrates that exquisite solid microspheres with spherical morphologies are included by the massive cenosphere particles created during the process of combustion.
Result with Discussion
The work’s goal is to give a complete analysis of existing research on various mix proportions in geopolymer mortar. The study involves the creation of various geopolymer mortar compositions in order to assess the impact of different variables. The FA concentration in the binder was kept constant at 50% in this investigation, while the fraction of RHA in the GGBS varied from 0% to 10% for each binder composition. Furthermore, different Na2SiO3 to NaOH concentrations (8M, 12M, and 16M) with two varied alkaline solution ratios (1.5 and 2) on the settling time, fresh characteristics, and CS of geopolymer concrete. Furthermore, we considered how these characteristics would alter under different curing circumstances. The experimental approach entails creating precise geopolymer concrete mixtures by ensuring the correct proportions of binder components, alkaline solutions, and curing conditions. The sections that follow provide a detailed discussion of the experimental approach used and the results obtained.
Figure 1 depicts the sieve analysis of fine aggregate. Here at lower limit (IS 383:2016) the percentage passing at a higher of 8, 35, 55, 75, 90 and 100 at IS sieve size of 0.4, 0.7, 2, 4, 7 and 10 respectively. Also for fine aggregate the percentage passing at a higher of 15, 45, 80, 95 and 100 at IS sieve size of 0.3, 0.6, 2, 5 and 7 respectively. Moreover at upper limit (IS 383:2016) the percentage passing at a higher of 10, 30, 60, 90 and 100 at IS sieve size of 0.2, 0.4, 0.8, 2 and 4 respectively.
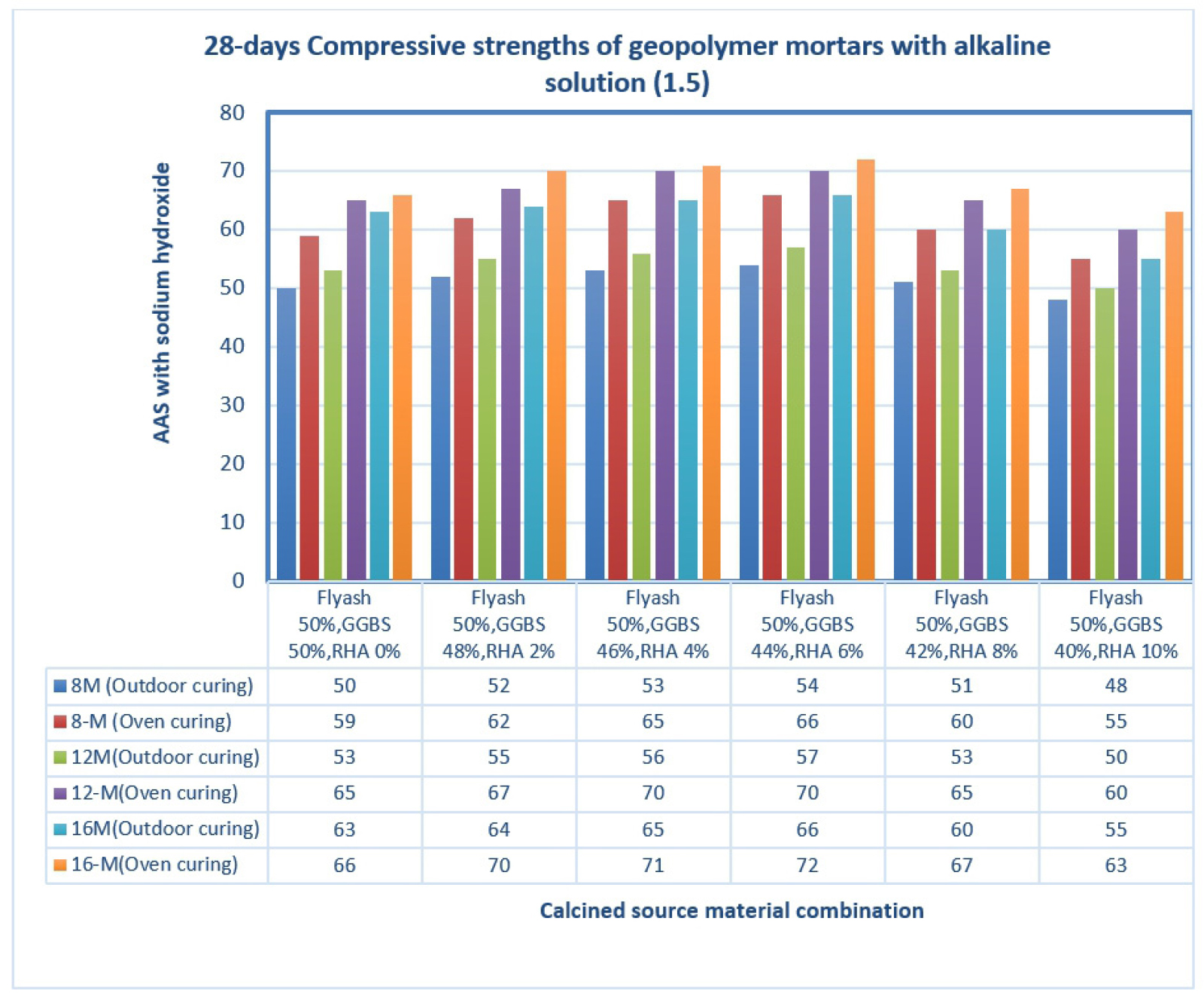
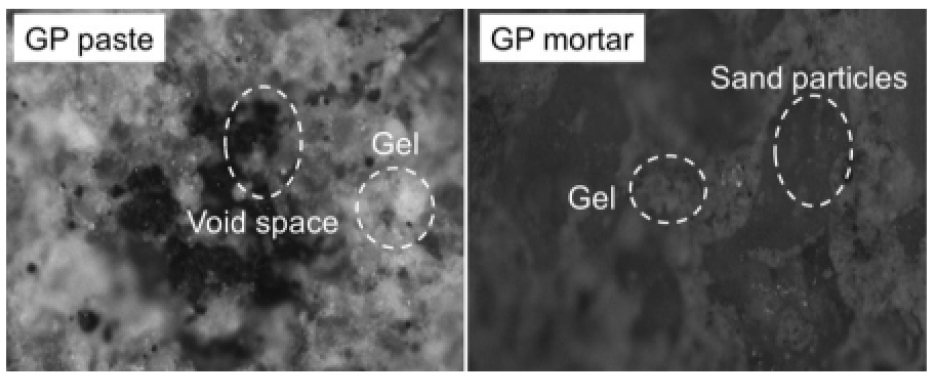
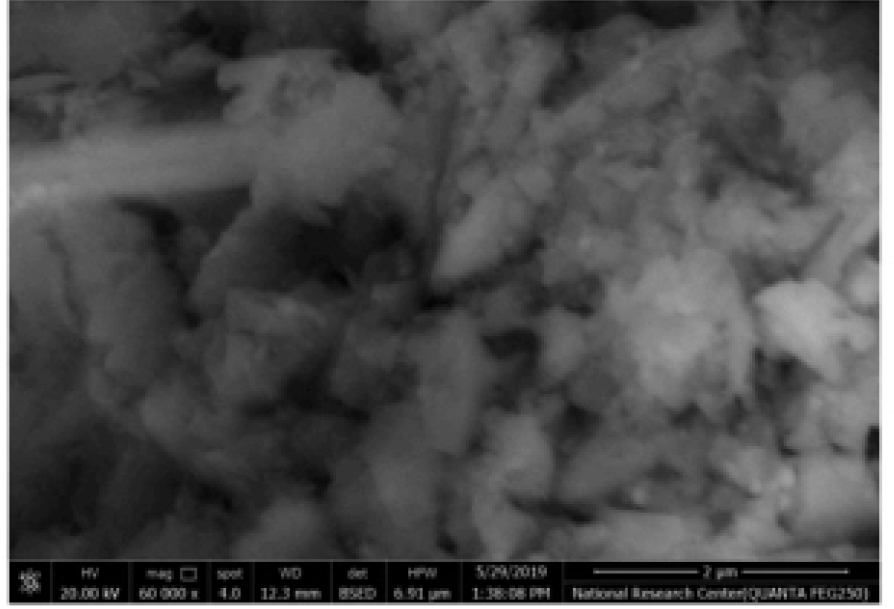
Figure 2 depicts the XRD analysis of binders (a) RHA (b) GGBS (c) fly ash. Here 2(a) shows the XRD analysis of RHA where the count attains 39, 25, 42, 20, 15 and 10 at a position of 0, 20, 30, 40, 50 and 60 respectively. Also 2(b) shows the XRD analysis of GGBS where the count attains 22, 60, 20, 10 and 15 at a position of 21, 28, 42, 50 and 62 respectively. Moreover 2(c) shows the XRD analysis of fly ash where the count attains 22, 90, 22, 10 and 15 at a position of 22, 28, 42, 50 and 62 respectively. Figure 3 depicts the analysis of 28 days geopolymer mortar compressive strengths (MPa) with an alkaline solution (1.5) with different 8M, 12M and 16M. Here addition of fly ash 50%, GGBS 44% and RHA 6% attains higher AAS sodium hydroxide while combining with calcined source material. The higher value obtained for 8M outdoor curing is 54, 8M oven curing is 66, 12M outdoor curing is 57, 12M oven curing is 70, 16M outdoor curing is 66, 16M oven curing is 72. Figure 4 depicts the analysis of 28 days geopolymer mortar compressive strengths (MPa) with an alkaline solution (2) with different 8M, 12M and 16M. Here addition of fly ash 50%, GGBS 44% and RHA 6% attains higher AAS sodium hydroxide while combining with calcined source material. The higher value obtained for 8M outdoor curing is 56, 8M oven curing is 68, 12M outdoor curing is 58, 12M oven curing is 72, 16M outdoor curing is 69, 16M oven curing is 74. Figure 5 depicts the SEM analysis of binders (a) SEM of RHA (b) SEM of GGBS (c) SEM of fly ash. According to the XRD graph the RHA (Figure 5(a)) was mainly in amorphous form due to the broad peak on 2θ angle of 220.The primary concentrations seen in the GGBS sample (Figure 5(b)) corresponded to calcite and quartz, with peak positions at 28, 35, and 37 degrees for calcite and 21 and 42 degrees for quartz. The XRD examination revealed that the primary prominent peak observed in fly ash (Figure 5(c)) corresponded to quartz, exhibiting a significant intensity at 2ϴ = 27°. Additionally, Mullet was found as the secondary major peak in fly ash, appearing at various 2ϴ values (17, 32, 33, 42, 50, and 61°). Figure 6 displays the magnifed image of S60 geopolymer mortar and paste specimens. The geopolymer mortar specimen has a solid, non-porous structure, but the geopolymer paste specimen has a lot of tiny pores. It is known that water molecules are extracted from the geopolymer matrix during the binder’s polycondensation process, creating a large number of micro-voids. Figure 7 and 8 illustrate the scanning electron microscopy (SEM) which was conducted for geopolymer samples after 28 days in oven curing. High mass structure accompanied by very low amount of pores and high homogeneity were recognised in Figure 7 compared Figure 8. This could be explained by the high concentration of sodium silicate in Figure 4, which results in an alkali-activated system and a high concentration of reactive silicate. These components interact with the dissolved calcium to form a high degree of polymerization and excessive C-S-H content (low Ca/Si ratio). As a result, the compressive strength values and the SEM results coincide.
The primary goal of this work is to explain the key aspects of GP composite paste, particularly its normal consistency (P) and setting time. This study is also responsible for evaluating how fresh materials perform in terms of their state, workability, and material strength. Extensive testing of GPC was carried out using a variety of trial-and-error methodologies. Throughout these methods, both compressive strength and deformation resistance were measured. Vicat’s device is the gold standard for measuring the standard consistency of cement. This technique is in accordance with the 1988 edition of IS 4031 (Part 4).Similarly, a complete study of the geopolymer material was carried out, using a method similar to that used when examining other materials. To achieve the desired consistency, alkaline solutions of various molarities (8M, 12M, and 16M, containing Na2SiO3 to NaOH were methodically prepared. These cures contained NaOH and Na2SiO3 as components. The IST and FST of the geopolymer paste were measured using a Vicat device. All of this complies with the requirements of IS 4031 (Part 5) (1988). The geopolymer paste setting times were studied using three distinct binder combinations: FA, pulverized granulated blast furnace slag, and RHS. This action was performed to improve exploration even further. In this study, the binder’s percentage of fly ash was kept stable at 50% while the RHA percentage in the GGBS was varied from 0% to 10%. Binders of various compositions were utilized, as well as alkaline solutions of different molarities (1.5 and 2). As a result, a time-consuming inspection of standard consistency, as well as the first and last setting of geopolymer paste samples, was substantially shortened. To ensure that all samples were the same size and consistency, the standard was set at 500 g in weight and 0.85 times the norm. Once the hardening process is complete, the mechanical properties of GPC mixtures will be the primary focus of this research. The full set was made up of 108 cubes that were 150 mm on all three sides. Compression tests were performed on these cubes at 3, 7, and 28 days, as stipulated by the IS 516 (1959) standard. The compression tests were carried out with remarkable precision utilizing Compressive Testing Equipment with a capacity of 2,000 kN. The performance profile of aggregate GPC was found to be similar to that of conventional OPC concrete in this investigation. A detailed analyses was conducted to determine the exact ratio of fine to coarse aggregate throughout the total aggregate, taking into account the medium’s inert qualities. Finally, the aggregate mixture with the ratio of 0.45:0.55 was chosen because it consistently provided the highest dry density when compared to the other aggregate mixtures. The most crucial aspect of this inquiry was discovering aggregates and solution components, as well as doing laborious calculations to estimate the quantities of each ingredient and their appropriate proportions. This procedure allowed for a complete and rigorous investigation of the geopolymer composite paste and its subsequent evolution into concrete, providing useful insights into the concrete’s mechanical properties and setting characteristics.
The Table 4 illustrates the X-Ray fluorescence analysis of fine RHA and OPC.
Table 4.
X-Ray Fluorescence analysis of Fine RHA and OPC
Conclusion
This experimental study used varying quantities of sodium hydroxide molarities (8M, 12M, and 16M) in the AAS as well as variations in binder content to determine the normal consistency, beginning, final setting time, and CS of 28 days. The ratio of binder to Alkali solutions 1.5and 2.0 were worked out with curing regimes outdoor and oven curing to determine the CS of GPM. The findings derived from the experimental inquiry are summarized as follows:
1.Using different amounts of sodium hydroxide (8M, 12M, and 16M) in the alkaline activator doesn’t seem to change the normal consistency of the geopolymer. Neither does using different amounts of fly ash, GGBS, and RHA as binder ingredients.
2.When GGBS and RHA are replaced with 0 to 10%, the geopolymer paste sets more slowly and has a higher normal consistency. This is because the amount of binder increases, especially RHA. When taking into account a composition of 50% fly ash, 40% GGBS and 10% RHA, this behaviour is more significant.
3.The proportion of NaOH to Na2SiO3 (1.5 and 2.0) does not appear to have a significant effect on the GP mortar’s consistency.
4.When the molarity of sodium hydroxide rises, the final setting time increases as well, from 8M to 16M in the alkaline solutions, particularly when the binder amount varies. The CS of the GP mortar rises when a higher molarity of sodium hydroxide (8M to 16M) is present in the AAS.
5.This is especially true when replacing GGBS with an increasing percentage of RHA, up to a 6% replacement rate. However, the CS of the geopolymer decreases when the replacement rate of GGBS with RHA exceeds 6%.
6.The geopolymer mortar’s CS significantly raises’ when the molarities of sodium hydroxide (8M, 12M, and 16M) change. It’s also important to emphasize that the compressive strength attained during oven curing is noticeably higher than that attained during ambient curing.