Introduction
Methodology
Preparation of Sulphuric Acid-activated Flower Waste (FWSAC) Adsorbent
Adsorbate Preparation
Adsorption Studies
Results and Discussion
FTIR analysis
FE-SEM analysis
Effect on pH
Effect on Contact Time
Effect on Initial concentration of dye
Effect on Adsorbent dose
Adsorption Isotherm Analysis
Adsorption Kinetic Study Analysis
Regeneration Study
Limitations and Scope for Future Work
Conclusion and Future Work
Introduction
Water is essential for all life forms, including ecosystems, communities, and agriculture. Water pollution has become more severe with the rise in population, urbanization, and industrial activity [1]. Though essential for economic growth, industries negatively affect the environment, especially water bodies. Industrial wastewater pollutes rivers and lakes, harming aquatic life and people living nearby [2]. The textile industry is one of the worst pollutants of all industries. It releases a lot of dyes, chemicals, and foul-smelling materials into the water. These pollutants make the water look unappealing, block sunlight in water bodies, and introduce harmful compounds like acids and toxins [3]. Many dyes are toxic to aquatic life, may cause skin irritation, and are even carcinogenic for humans [4]. Dyes are coloured substances that bond chemically with the materials they colour [5]. They can be natural, like those from plants, minerals, or synthetic. Synthetic dyes are made from chemicals derived from coal tar [6]. Methylene blue, a synthetic dye, is used in the textile, paper, and medical industries to colour materials like cotton, wool, and hair [7]. However, it is toxic to human health, causing nausea, breathing problems, eye irritation, and diarrhoea. Methylene blue also doesn’t break down easily and tends to build up industrial wastewater, posing a severe environmental threat [8]. The adsorption process, excluding such cationic dye from aqua, has proven effective, economical, and ecologically sustainable. The process of adsorption utilizes porous media to capture and remove substances from water, and unlike other methods, it doesn’t produce harmful by-products [9, 10]. The significance of methylene blue (MB) removal lies in its prevalence as a toxic dye in textile wastewater, which poses severe environmental and health risks. Effective treatment of MB is crucial for mitigating pollution and ensuring safe water resources [11]. The applications of MB removal extend to improving water quality in industrial processes, enhancing ecological health, and complying with environmental regulations [12]. Effective removal methods are essential to mitigate these impacts, making agricultural adsorbents like peanut and rice husk valuable alternatives for wastewater treatment, and promoting sustainable practices while addressing the challenges posed by dye contamination [13].
However, an effective adsorbent-activated charcoal is quite costly. Adsorbents made from flower waste provide one of the promising solutions as low-cost alternatives [11, 12]. Marigold flowers, widely used in festivals and temples in India, create a lot of floral waste; disposing of this is challenging. Converting marigold flower waste into an adsorbent makes it possible to remove dyes from wastewater in an affordable and environmentally friendly way [13]. This approach highlights the potential for flower waste to help purify water, promoting a greener environment.
Methodology
Preparation of Sulphuric Acid-activated Flower Waste (FWSAC) Adsorbent
The steps involved in preparing an adsorbent from Marigold flowers collected from a temple in the Nagpur District, Maharashtra, India, were as follows. The flowers were thoroughly rinsed with deionized water to remove dirt, impurities, etc., and then sun-dried. They were dried in a hot oven at 105-110°C to ensure complete moisture removal for 24 hours. The dried flowers were placed in a crucible and heated at 550°C for 2 hours in a muffle furnace. After carbonization, the resulting ash was cooled to ambient temperature in a desiccator. The ash was then activated by mixing it with sulphuric acid (H2SO4) [11]. After activation, it was neutralized by washing with deionized water. To remove any remaining moisture, the material was dried again in an oven at 105-110°C. The dried material was then ground with a mortar and pestle into a fine powder with a particle size between 210-300 μm. The moisture free fine-activated powder (FWSAC) was then use in adsorption studies.
Adsorbate Preparation
A required stock solution of methylene blue (MB) was prepared by dissolving a suitable amount of the dye in distilled water. From this stock solution, multiple diluted concentrations were created at 10 ppm, 20 ppm, 30 ppm, 40 ppm, and 50 ppm. To facilitate the performance of the experiment, a calibration curve was constructed using these specified concentrations of the stock solution [12].
Adsorption Studies
Equilibrium Studies
The current work was conducted through batch adsorption tests at a constant temperature of 30°C and a stirring speed of 150 rpm. The studies focused on crucial adsorption parameters, including pH, contact time, the adsorbate's initial concentration, and the adsorbent's dose. To evaluate these parameters, one variable was altered while keeping the others constant, allowing for the determination of optimal conditions in each experiment. After each cycle, the absorbance of the solutions was measured using a UV-visible spectrophotometer at a wavelength of 663 nm [12]. All formulas are shown in Equations from 1 to 8 as shown in Table 1.
Table 1.
Equations for Adsorption Analysis
Isotherm Studies
Adsorption isotherms can quantify contaminants adhered to the surface of the adsorbent at an equilibrium concentration and constant temperature. The adsorption isotherm primarily governs the interaction between the adsorbent material and the adsorbate and estimates the adsorption capacity. Adsorption models are significant in explaining the adsorption mechanism [14].
Kinetic Models
Kinetic studies describe the retention or release of the adsorbate from the aqueous dilution onto the solid porous adsorbent. Another aspect of kinetic examination is the study of the variables influencing the rates, such as pH, the dose of the adsorbent, and flow rate. An in-depth analysis of the experimental conditions is necessary to assess their potential impact on the degree of the adsorption reaction and achieve equilibrium adsorption within a suitable duration. While numerous kinetic models can be used to describe the adsorption process, most of them are not practical due to their mathematical intricacy. However, based on the literature, the Lageregen and pseudo-second-order equations are the most frequently used models for wastewater adsorption [7].
Results and Discussion
FTIR analysis
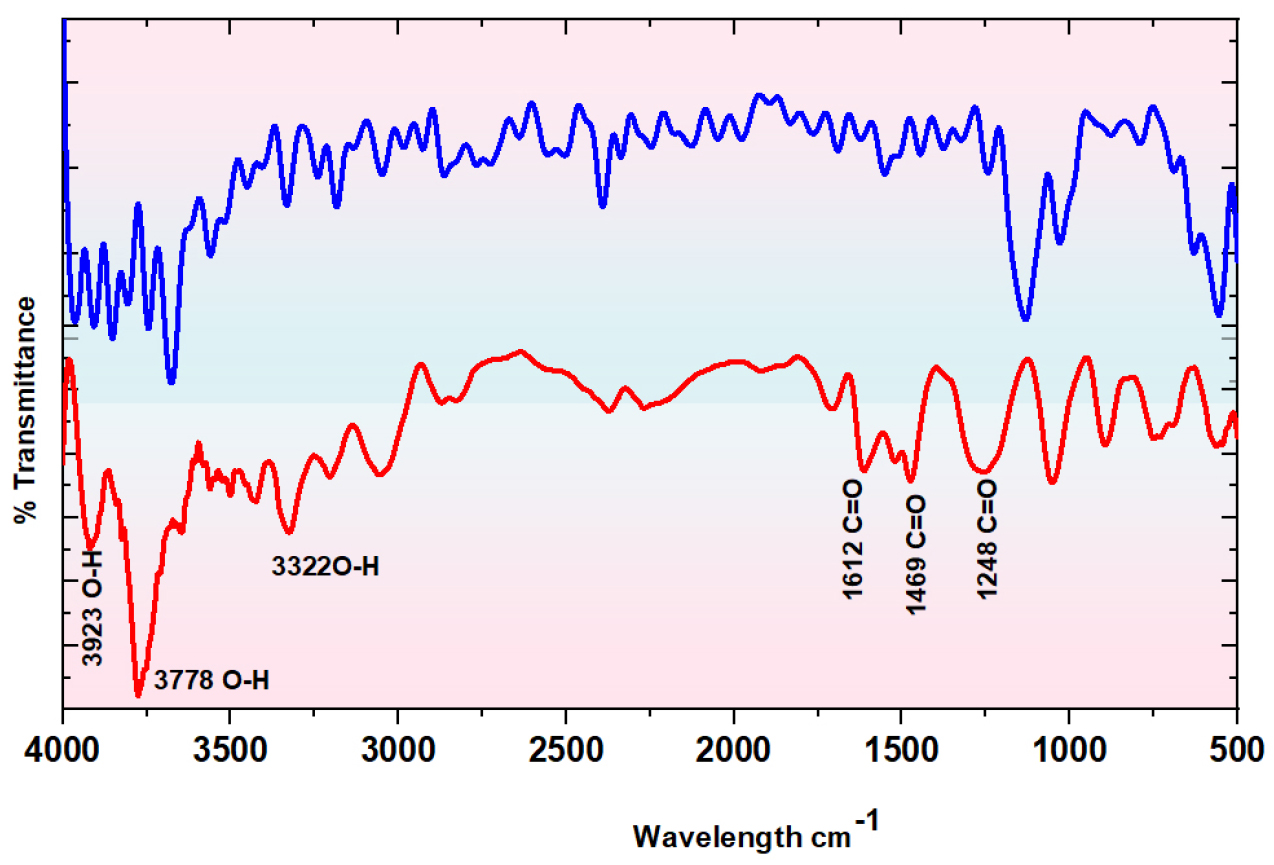
FTIR analysis has been carried out to examine the presence of functional groups accountable for the adsorption process. FTIR spectroscopy observations are presented in Figure 1 confirmed the existence of various functional groups in the adsorbent, as shown by peaks observed before and after the MB adsorption. The noticeable changes in the spectrum imply that MB, adhering to FWSAC, indicates the occurrence of effective functional groups due to adsorbate-adsorbent interaction, ultimately reflecting improved adsorption process. Variations significantly influence ion exchange [15] and dyes' bonding throughout biosorption in maximum values. The presence of alcohol and phenol causes hydroxide (O-H) group stretching, as observed in the broad spectral bands (3322 - 3923) cm-1. The stretching occurs as a result of the adsorption of MB on FWSAC. The fluctuations at (1248-1612) cm-1 can be attributable to the methyl group's C=O bond elongation. Increased oxygenated groups on FWSAC may result from acid oxidation, which could enhance the dye's capacity to bind through biosorption [16].
FE-SEM analysis
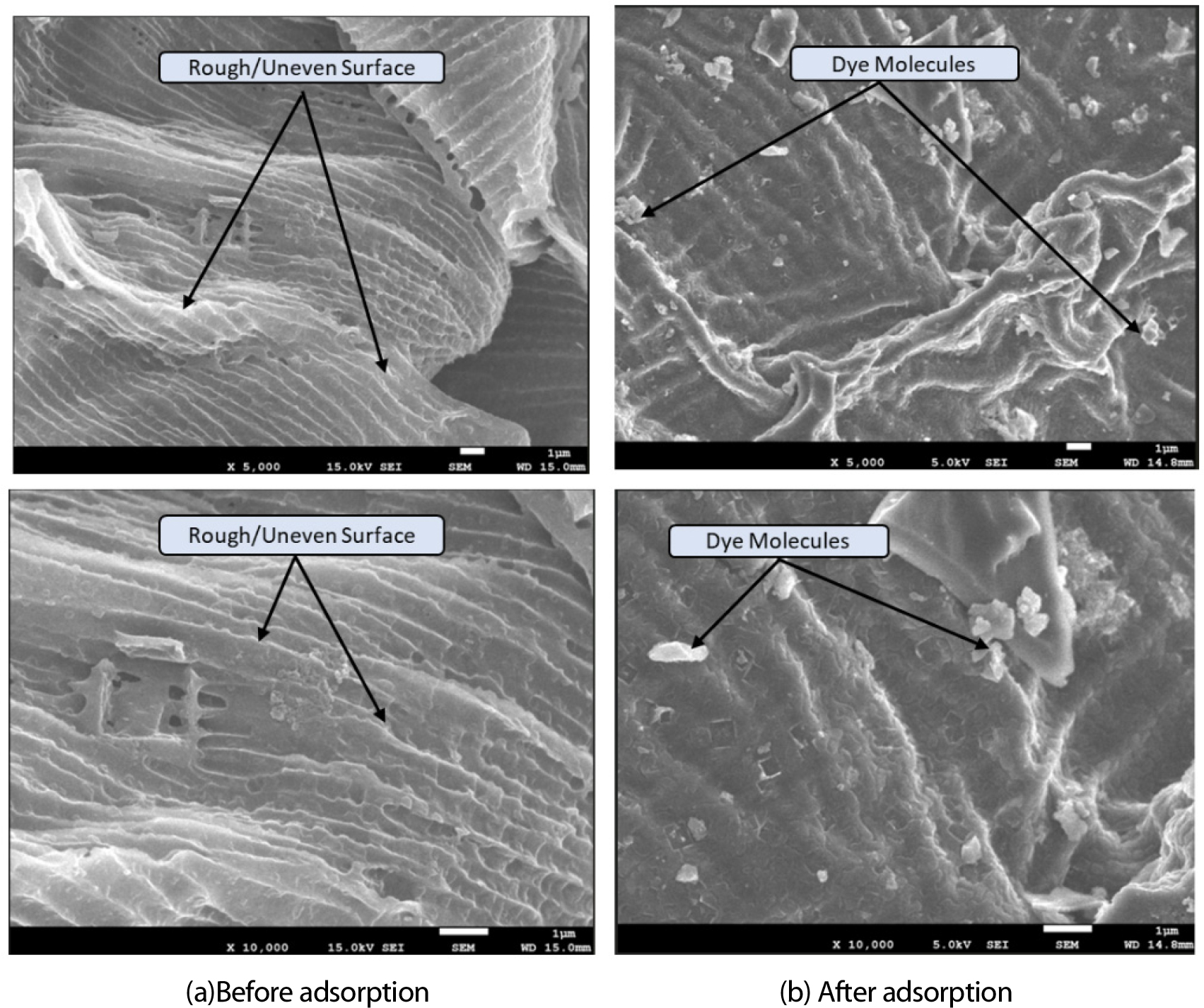
Figure 2 (a) and (b) provide the FE-SEM images indicating the microstructural analysis of FWSAC for the adsorption of MB for pre- and post-adsorption, respectively. The uneven, rough structure of the FWSAC adsorbent is likely because of the sulphuric acid treatment [1], which possibly ends in improving the adsorption extent of dye molecules on the adsorbent. The adsorbents activated through acid shows high adsorption capacities and more efficiency in fastening contaminants due to refined surface characteristics and improved ion exchange and active adhering spots [1]. The post adsorption analysis indicates comparatively smoother surface, suggesting adherence of the dye molecule to the adsorbent surface [17].
Effect on pH
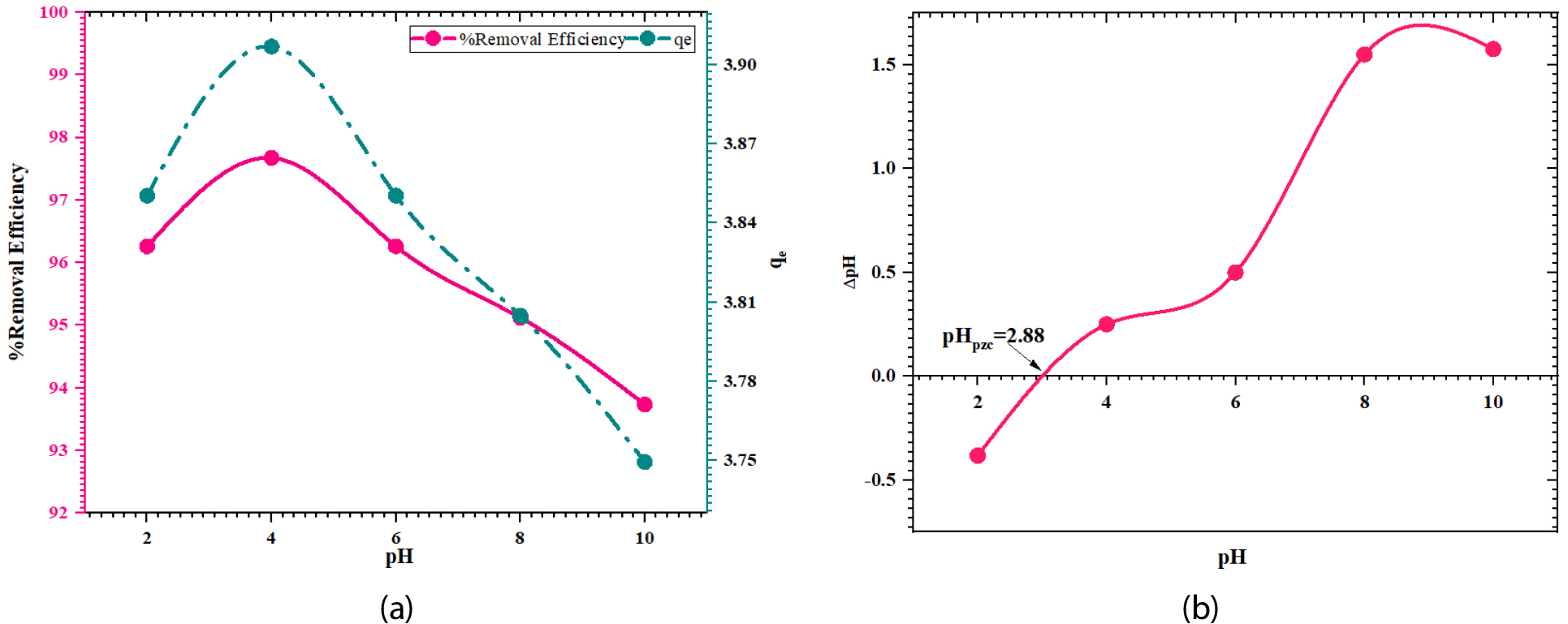
Using FWSAC adsorbent, the impact of pH on the adsorption of methylene blue was investigated under a 90-minute reaction duration: pH range: 2 to 10 adjusted using 0.1M HCl and 0.1M NaOH; temperature: 30±0.5°C; agitation speed of 150 rpm; adsorbent dosage:
0.25 g/100mL; adsorbate initial concentration: 10 mg/L. With an adsorption capacity ranging from 3.85 mg/gm to 3.90 mg/gm, the adsorption rates varied from 96.26% in the pH 4 acidic region to 93.73% in the pH 10 alkaline range, as illustrated in Figure 3 (a). The physicochemical characteristics of the pollutants, the functional hydroxyl group of the adsorbent, the surface charges of the sorbents, and interactions with the concentration of competing ions are some possible causes for the maximum reduction of 96.26% at pH 4. It was discovered that the FWSAC adsorbent had a negative surface charge, with a point of zero charge of 2.88 (pH>pHpzc), as shown in Figure 3 (b). This suggests that the adsorbent and the cationic MB dye molecule are electrostatically attracted, leading to maximum removal effectiveness at a pH of 4 [18].
Effect on Contact Time
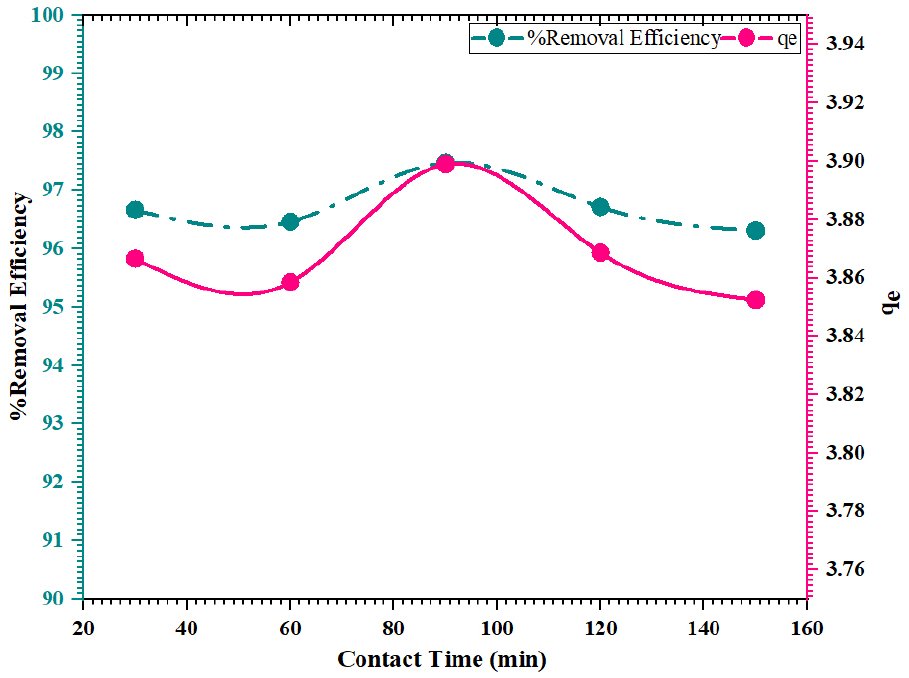
Using FWSAC adsorbent, the impact of contact time on the adsorption of methylene blue was investigated under a reaction duration of 30-150 minutes with an optimum pH of 4, temperature 30±0.5°C, agitation speed of 150 rpm, adsorbent dosage of 0.25 g/100mL, and adsorbate initial concentration of 10 mg/L. Figure 4. demonstrates that the adsorption percentage increased from 96.64% to 97.47% throughout a contact period of 30 to 90 minutes. The adsorption uptake increased from 3.86 mg/g to 3.89 mg/g respectively. This occurred because there were vacant binding sites on the surface of the adsorbent. The adsorption rate decreased to 96.31% when the contact period exceeded 90 minutes, mostly because the quantity of accessible adsorption sites declined. This can be due to the significant increase in the resistance of the contact forces between the adsorbate and active adsorptive sites [19, 20, 21]. It was determined that the optimal duration for conducting an additional trial was 90 minutes.
Effect on Initial concentration of dye
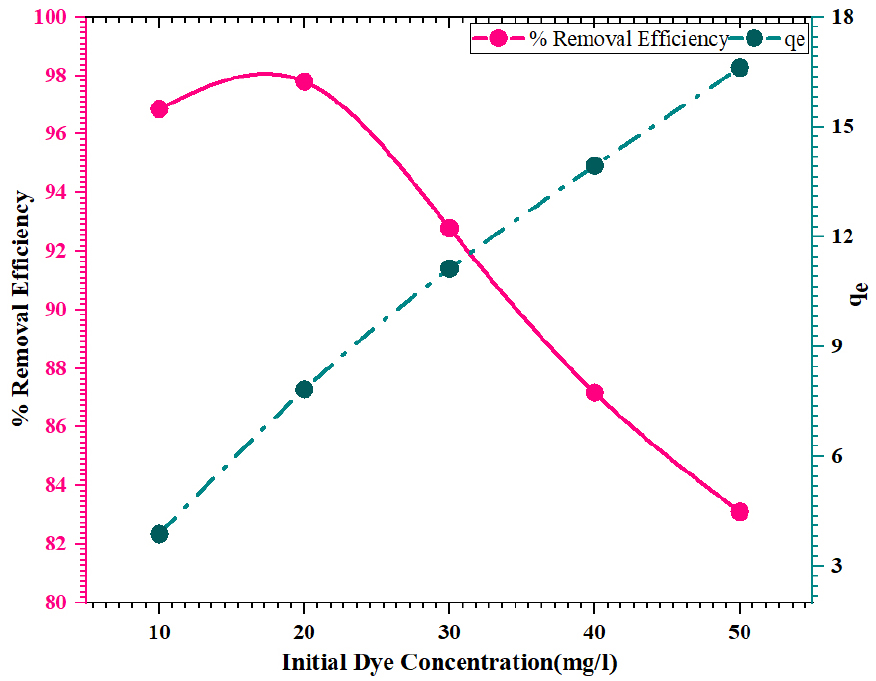
Using FWSAC adsorbent, the impact of the initial concentration of dye on the adsorption of methylene blue was investigated with varying adsorbate initial concentrations from 10-50 mg/L under the optimum reaction duration of 90 minutes, optimum pH of 4, temperature 30±0.5°C, agitation speed of 150 rpm, and adsorbent dosage of 0.25 g/100 mL. The adsorption capacity increases from 3.87 mg/g to 16.62 mg/g as the initial concentration of methylene blue rises from 10 mg/L to 50 mg/L, as depicted in Figure 5. This may lead to the increment in the driving force required to subdue the resistance to the mass transfer of dye between the solution and solid media [22]. Augmenting the initial adsorbate concentration may enhance the adsorption process by accelerating the adheration frequency of the dye and the bio-adsorbent. The removal efficiency was 96.86% at a concentration of 10 mg/L and improved to 97.81% at 20 mg/L. The dye removal efficiency fell from 97.81% to 83.10%, with an increase in concentration from 20 to 50 mg/L. This phenomenon occurs when the adsorption sites become saturated as dye fragments accumulate on the surface of the adsorbents.
The experiment results indicated that 20 mg/L was the optimal concentration for further study. The dye removal efficiency fell from 97.81% to 83.10%, with an increase in concentration from 20 to 50 mg/L. This phenomenon occurs when the adsorption sites become saturated as dye fragments accumulate on the surface of the adsorbents. The experiment results indicated that 20 mg/L was the optimal concentration for further study.
Effect on Adsorbent dose
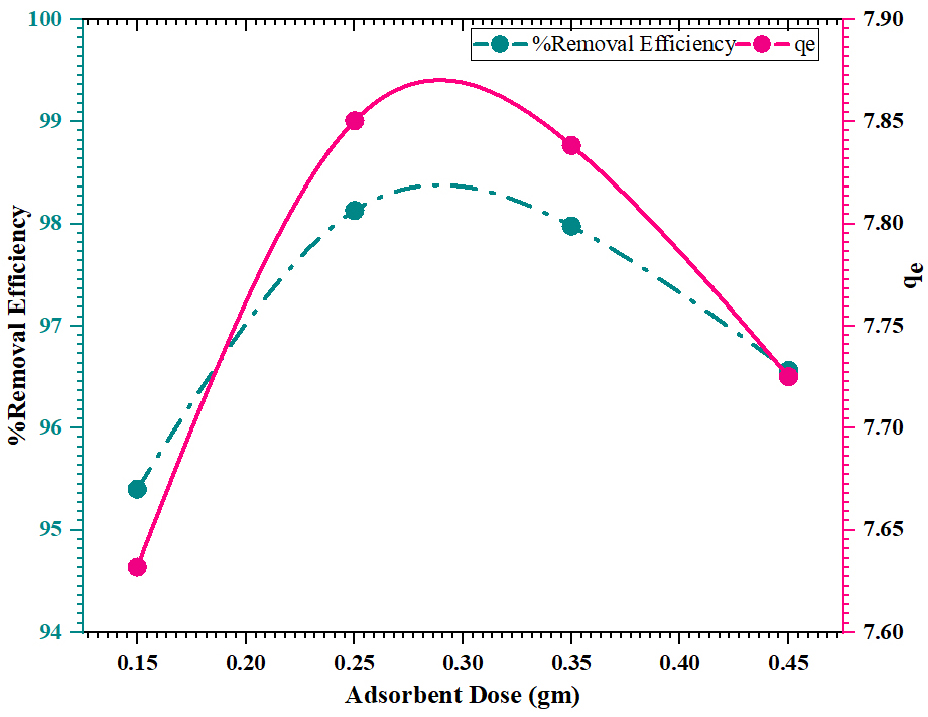
Using FWSAC adsorbent, the impact of contact time on the adsorption of methylene blue was investigated at varying adsorbent doses of 0.15 gm/100mL to 0.45 gm/100 mL under the optimum reaction duration of 90 minutes, with an optimum pH of 4 for an optimum dye solution of 20 mg/L, a temperature 30±0.5°C, and an agitation speed of 150 rpm. Figure 6, shows that the adsorption removal efficacy increases from 95.40% to 98.13% when the dose is increased from 0.15 gm to 0.25 gm and then slightly drops to 96.56% at 0.45 gm. The increase in removal rate when the initial adsorbent dose rises results in the expansion of the surface area of media and the increased presence of active adsorption spaces.
Adsorption uptake reduces from 12.72 mg/g to 4.29 mg/g with an increase in adsorbent dosage from 0.15 gm to 0.45 gm. The decrease in adsorption capacity could be caused by the saturation of adsorption sites, an increase in accessible sites for adsorption, or the aggregation of adsorbent particles, leading to a diminution in total surface area [23].
Adsorption Isotherm Analysis
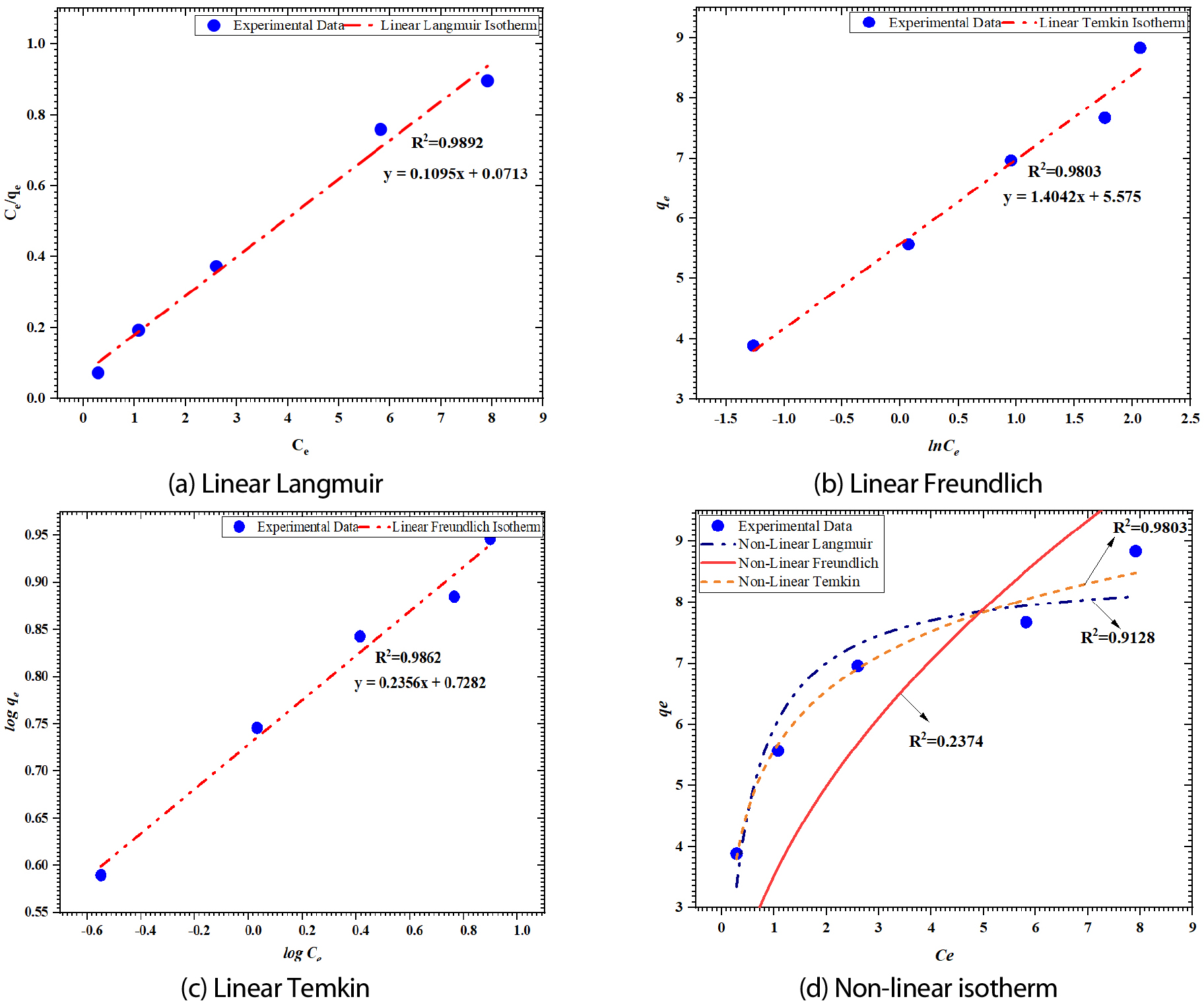
Figure 7 (a), (b), and (c), Table 2, and the equations and plots represented indicate the experimental outcomes were well fitted to the Langmuir isotherm, as opposed to the Freundlich and Temkin models. This is likely because biochar has many surface-adhering sites, enhancing the affinity of MB dye on the material [24, 25]. The calculated RL values in Table 3 for FWSAC varied between 0.927 and 0.810, showing a significant adsorption capacity at dye concentrations ranging from 10 to 30 mg/g. The particles adhered effectively to the adsorbent surface shown in Figure 7 (a), confirming that the Langmuir isotherm promotes MB adsorption. Figure 7 (b) illustrates that the Freundlich isotherm parameters were also calculated by graphing ln qe vs ln Ce. The model's findings show that R2 is 0.9862 and Kf is 5.348 mg/g. The Freundlich adsorption (1/n) intensity or 0.235 < 1 achieved under these conditions suggests promising adsorption. Figure 7 (c) further shows that when plotted against qe versus ln Ce, the Temkin isotherm model exhibits an excellent linearity fit with correlation coefficients R2= 0.9803. As illustrated in Figure 7 (d), the nonlinear forms of the three isotherm models were compared. Results from the regression analysis showed that the MB adsorption on the FWSAC biosorbent was best fit by the Temkin model (R2=0.9803), which was more accurate than the Langmuir model (R2=0.912). The Freundlich model showed less agreement with the experimental data (R2=0.2374).
Table 2.
Adsorption isotherm parameters for MB adsorption on FWSAC
Table 3.
Separation factor (RL) at varying dye concentrations for FWSAC
| Dye Concentrations (Co) (mg/L) | 10 | 15 | 20 | 25 | 30 |
| RL | 0.927 | 0.895 | 0.865 | 0.836 | 0.810 |
Adsorption Kinetic Study Analysis
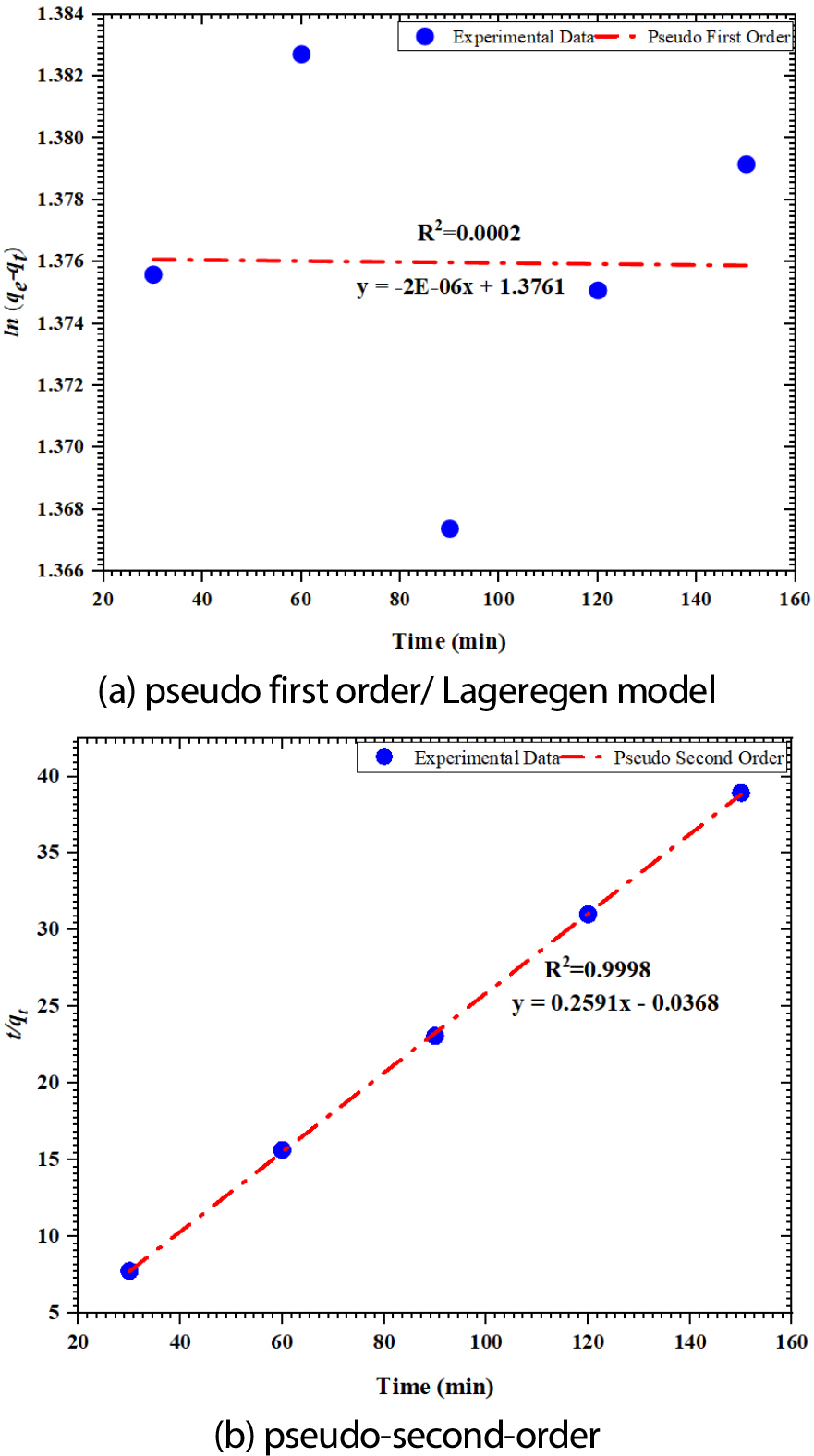
Figure 8 (a) and (b) represent a graphical interpretation of the Lageregen and pseudo-second-order models, respectively, and Table 4 provides the calculated kinetic parameter-based coefficient of correlation (R2) for both models using Equations 7 and 8. The linear fit for the Lageregen kinetic model is deprived, with an R² value of 0.0002. This indicates that this model does not adequately describe the adsorption process. Plotting (t/qt vs. t) for the pseudo-second-order kinetic model results in a straight line with a high correlation coefficient (R² > 0.999), indicating a better fit. The calculated equilibrium adsorption capacity (Cecal = 3.855 mg/g) is very close to the experimental value (keep = 3.859 mg/g), further supporting the model's accuracy. Comparing the two kinetic models allows one to conclude a pertinent interaction between the MB adsorption on FWSAC and the pseudo-second-order kinetic model. This implies that the addition of chemical adsorption may regulate the adsorption process [25].
Table 4.
Validation of Adsorption Kinetic model for MB adsorption on FWSAC
| Kinetic models | Parameters | Numerical Value |
| Pseudo-first order | qeexp | 3.95±0.006 |
| qecal | 1.423 | |
| k1 (min-1) | -1.33E-08 | |
| R2 | 0.0002 | |
| Pseudo-second order | qeexp | 3.859±0.158 |
| qecal | 3.855 | |
| k2 (g/mg min) | 1.824 | |
| R2 | 0.999 |
Regeneration Study
Non-regenerative adsorbents become secondary pollutants become once discarded [26]. Dye-containing adsorbents can be burnt after removal, eliminating waste disposal difficulties [27]. The regeneration potential of created bio-adsorbents was evaluated using the method described by [28]. Upon the completion of the biosorption process under optimal conditions, the dye-loaded adsorbent subsequently dried [29, 30, 31, 32, 33, 34, 35, 36]. Eluting agent viz., 50 mL 0.1 M (each of) HCl and NaOH was added to the dye-loaded adsorbent in separate conical flasks. A 2-hour desorption operation was conducted using an orbital shaker, identical to the biosorption examination. The bio-adsorbent was meticulously washed with deionized water after desorption and then oven-dried. The efficiency of removing adsorbate was evaluated using the regenerated adsorbents [28].
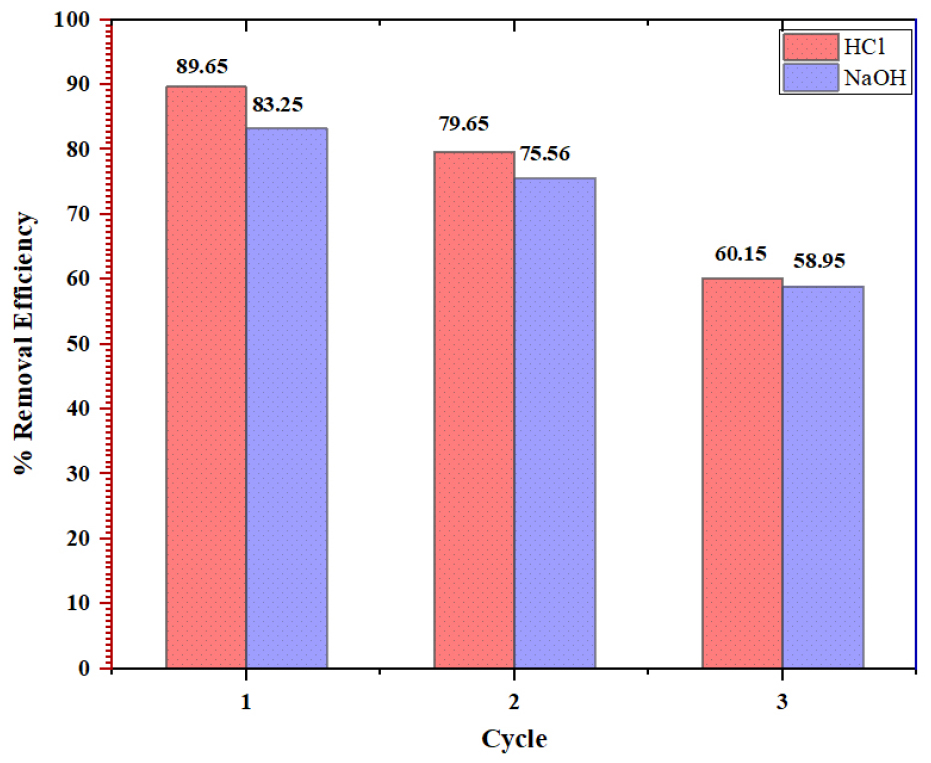
Using hydrochloric acid as an eluting agent in the first cycle led to an MB removal rate of 89.65%, while in the second cycle, it was 79.65%. The removal rates were 83.25% and 75.56% when NaOH was used as the eluting agent, as shown in Figure 9. After three cycles, the HCl reusability declined to 60.15%, and the NaOH reusability dropped to 58.95%. The saturation level of the adsorbent solution may affect the desorption process.
Limitations and Scope for Future Work
The suitability of FWSAC for the effective removal of MB dye from water systems is reflected as an economical and ecologically friendly adsorbent. An economical waste management strategy is to use chemically or physically activated agricultural waste in place of more expensive, conventional commercial adsorbents. The process cost is directly impacted by the absorbent cost. Commercial activated carbon is more expensive even if it is more effective at elimination. Therefore, the use of locally accessible, reasonably priced materials with carbon-like absorption capabilities is urgently needed. Before large-scale adoption, further investigation is necessary to create practical strategies for producing adsorbents from waste materials, with a focus on pilot plant and commercial viability. The understanding of the behavior, effectiveness, and possible issues with the adsorbent in real-world applications will be improved as a result of these studies. A comprehensive life-cycle assessment should be required to evaluate the overall cost-effectiveness and environmental implications before deciding on the implementation of these strategies.
Conclusion and Future Work
The present work utilized sulphuric acid-activated marigold flower adsorbent to remove methylene blue dye from an aqueous solution. The following conclusions were derived from the analysis. The optimal parameters for MB dye adsorption about dye removal using H2SO4-activated marigold flower waste (FWSAC) were determined by equilibrium tests. The removal effectiveness of over 90% was achieved with 90 90-minute contact duration with an initial adsorbate solution of 20 mg/L and adsorbent dosage of 0.25 at a pH of 4. Adsorption isotherm tests with Langmuir, Freundlich, and Temkin models revealed that the isotherm model best characterized the biosorption behaviour toward MB elimination. Both the linear and non-linear forms of the model's coefficient of regression indicate monolayer adsorption, having a maximum adsorption capacity (max) of 14.02 mg/g with R2 =0.9892 (linear form) and 8.54 with R2=0.912 (non-linear). The system exhibits the Temkin isotherm model (FWSAC) in its non-linear form with R2 = 0.9803, suggesting significant interactions between the adsorbate and the material. The FWSAC closely adheres to the pseudo- second-order kinetic model for MB adsorption. This indicates that the model accurately describes the adsorption process and fits well with the experimental data. The regeneration and desorption experiments demonstrated that the FWSAC removed the MB dye successfully. This research establishes a basis for the enhancement of marigold-derived activated carbon (FWSAC) in wastewater treatment applications. Future research may investigate alternative activation methods, such as physical or chemical agents like ZnCl2/KOH, to improve adsorption capacity and cost-effectiveness.